Researchers have unraveled a decades-old geochemical mystery by pinpointing the mechanism behind enigmatic mass-independent Sn isotope fractionation (Sn-MIF), transforming this industrial metal into a powerful tracer for Earths ancient atmosphere. Published in PNAS, a team from Nanjing University demonstrates that magnetic Sn isotopes (115Sn, 117Sn, 119Sn) fractionate dramatically under ultraviolet (UV) light via the magnetic isotope effect (MIE) - a phenomenon where nuclear spin influences chemical reactivity. Crucially, this signature vanishes under modern sunlight due to ozone shielding, positioning Sn-MIF as a potential tracer for reconstructing environments on early Earth and other planets.
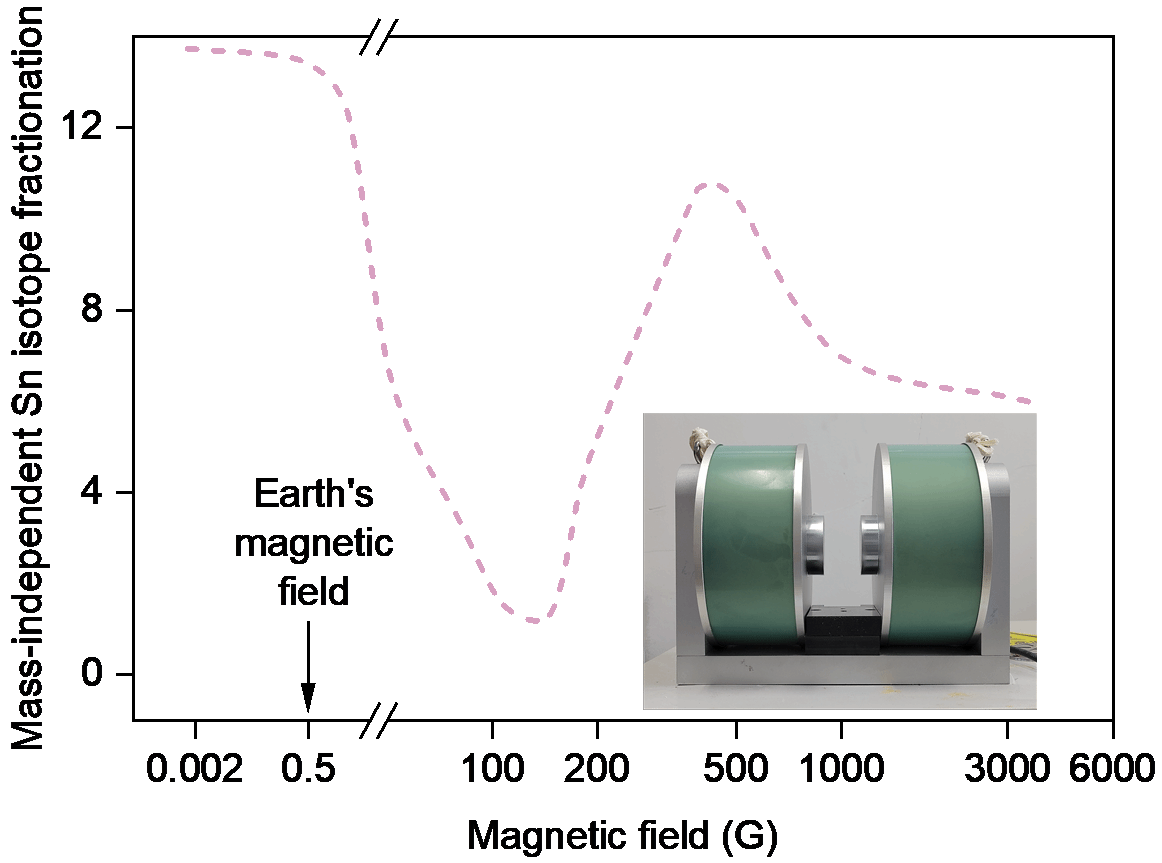
Relationship between mass-independent Sn isotope fractionation and magnetic field strength in photolysis experiments. Lower right: Self-designed magnetic field device.
The isotope puzzle solved
For decades, the origin of Sn-MIF signatures in meteorites and laboratory studies remained contentious: were Sn-MIF caused by nuclear volume effects, magnetic isotope effect, or both? Now, Professor Weiqiang Li's team settled the debate through meticulously designed experiments. The study leveraged Nanjing University’s state-of-the-art Nu Sapphire 1700 mass spectrometer, which enabled high-precision analysis of low-abundance 115Sn. By conducting photolysis experiments with UV lamps under tunable magnetic fields, they detected significant Sn-MIF exclusively in odd Sn isotopes with ratios matching nuclear magnetic moments. They also found that Sn-MIF shown non-monotonic relationship with magnetic field strength. Non-magnetic isotopes showed no Sn-MIF, definitively ruling out nuclear volume effects.
Under UV light mimicking an oxygen-poor Archean Earth, magnetic Sn isotopes fractionate significantly, up to 21‰ in Δ115Sn, solely due to the magnetic isotope effect (MIE). This phenomenon occurs when magnetic Sn nuclei affected unpaired electrons in radical pairs, shifting chemical pathways. Crucially, the team captured this process: Electron spin resonance spectroscopy detected methyl radicals (•CH3) during the reaction, while adding radical-trapping agents (DMPO) suppressed Sn-MIF.
Sunlight's contrasting role
When natural sunlight replaced UV light, Sn-MIF disappeared entirely, despite significant mass-dependent fractionation. Why? Modern Earth’s ozone layer blocks short-wave UV (<290 nm), the very radiation needed to birth radicals. Sn isotopes carry a UV fingerprint: No ozone means no Sn-MIF, making it a binary switch for atmospheric oxygen.
Why it matters for Earth and beyond
This discovery unlocks new ways to probe planetary evolution. Before the Great Oxidation Event (GOE), unfiltered UV reached Earth’s surface, imprinting Sn-MIF into minerals. Post-GOE, the signal switched off, making Sn isotopes a unique chronometer for the rise of oxygen. The team now eyes Archean shales and banded iron formations as "time capsules" of Sn.
Source:
She, J.-X., Li, W., Zhang, S., Gu, C., Chen, X., Zheng, H., Xu, C., Liu, W. (2025). Sensitivity of mass-independent Sn isotope fractionation to UV radiation and magnetic fields. Proceedings of the National Academy of Sciences, 122(24).https://doi.org/10.1073/pnas.2504065122
